Brown adipose tissue development & involution
Obesity is a global epidemic. The excessive fat accumulation in white adipose tissue (WAT) and visceral organs leads to chronic, low-grade inflammation, which is a driving factor for many diseases, including type 2 diabetes, hypertension, cardiovascular disease, and some types of cancers.
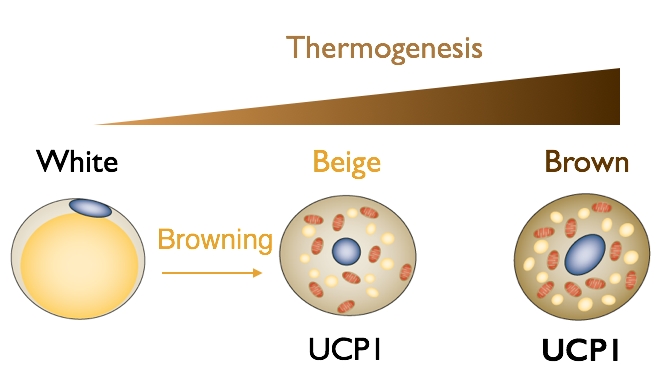
In contrast to classic WAT, brown adipose tissue (BAT) and “brown-like” beige adipocytes in WAT burn fat and dissipate chemical energy as heat (thermogenesis). In humans, BAT prevelance is associated with cardiometabolic health. There are two spatiotemporally distinct phases of BAT ontogeny: fetal development of interscapular & perirenal BAT depots and postnatal appearance of dispersed BAT in supraclavicular and perivascular regions. We currently use mouse as the model to study the adipocyte progenitors and metabolic impact of adult BAT.
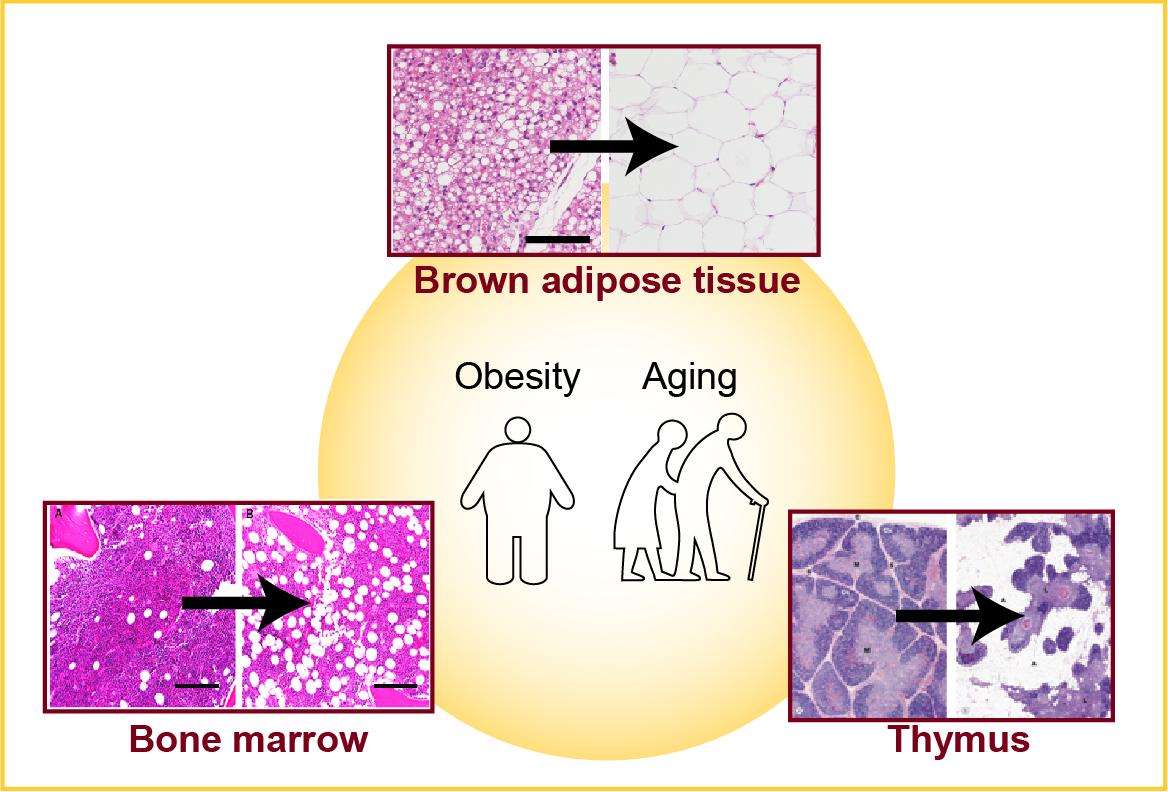
Efforts to recruit or activate thermogenic tissues have been overshadowed by the fact that BAT in humans undergoes age-dependent involution or atrophy. Understanding the involution process of BAT can provide insight into preserving or regenerating the tissue to treat obesity-related comorbidities. We use integrated approaches, including comparative physiology, next generation single cell-omics, and functional mouse genetics, to identify tissue-intrinsic (e.g. stem cell deficiency) and -extrinsic (e.g. inflammation) mechanisms that govern the age-associated BAT involution.
Adipose tissue associated with lymphoid organs
With obesity and aging, white adipocytes also accumulate within the primary lymphoid organs including bone marrow and thymus and the secondary lymphoid organs such as lymph nodes. Excess fat deposition is associated with the functional decline of the skeletal and hematopoietic systems, causing bone loss, marrow adiposity, inflammation, and immunosenescence. Several investigations are ongoing in the lab to determine the stem cell identity, regulatory signals, and functional impact of lymphoid organ-associated adipose tissue during obesity and aging.
Related publications:
1. Huang Z, Gu C, Zhang Z, Arianti R, Swaminathan A, Tran K, Battist A, Kristof E, Ruan HB. (2023). Supraclavicular brown adipocytes originate from Tbx1+ myoprogenitors. PLOS Biology. 21(12): e3002413. doi: 10.1371/journal.pbio.3002413.
2. Zhang Z, Huang Z, Awad M, Elsalanty M, Cray J, Ball LE, Maynard JC, Burlingame AL, Zeng H, Mansky KC, Ruan HB. (2023). O-GlcNAc glycosylation orchestrates fate decision and niche function of bone marrow stromal progenitors. eLife. 12:e85464..doi: 10.7554/eLife.85464.
3. Huang Z, Zhang Z, Moazzami Z, Heck R, Hu P, Nanda H, Ren K, Sun Z, Bartolomucci A, Gao Y, Chung D, Zhu W, Shen S, Ruan HB (2022). Brown adipose tissue involution driven by progressive restriction in progenitor competence. Cell Reports. 39(2): 110575.DOI: 10.1016/j.celrep.2022.110575.
4. Zhang Z, Huang Z, Ong B, Sahu C, Zeng H, Ruan HB. (2019). Bone marrow adipose tissue-derived stem cell factor mediates metabolic regulation of hematopoiesis. Haematologica. 104(9):1731-1743.